Abstract
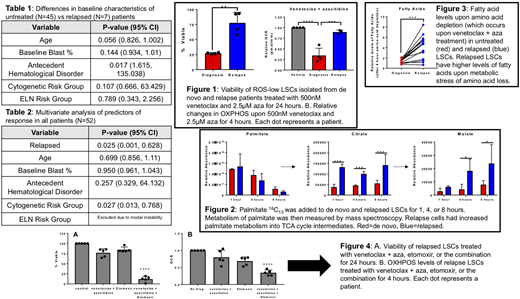
Effective targeting of the acute myeloid leukemia (AML) leukemia stem cell (LSC) population may allow for deep, durable remissions and curative potential. In older, newly diagnosed AML patients who are not candidates for induction, venetoclax + azacitidine (aza) targets specific metabolic vulnerabilities of LSCs, resulting in very promising clinical outcomes. In our single institution experience treating 45 previously untreated AML patients with venetoclax + aza both in the context of the multi-institutional study NCT02203773 (N=33) and with off-label use (N=12), 36/45 (80%) achieved a complete remission (CR) or CR with incomplete count recovery (CRi). In the relapsed/refractory (R/R) setting, the efficacy of venetoclax + aza has been reported to be significantly worse. In our single-institution off-label experience (N=7), only 1/7 (14%) R/R patients had a CR/CRi (p=0.005 compared to the untreated group). R/R and untreated patients had similar baseline characteristics, although more R/R patients had an antecedent hematological disorder (Table 1). Multivariate analysis showed cytogenetic risk and R/R disease as the sole predictors of response to venetoclax + aza (Table 2). In light of existing data regarding biological changes that occur in LSCs after treatment and subsequent relapse, we aimed to determine whether laboratory analysis of LSCs from patients treated with venetoclax + aza would show differential sensitivity to this therapy in the up-front vs R/R setting that could help to explain the different clinical activity.
We have previously shown that LSCs from untreated patients are uniquely reliant on oxidative phosphorylation (OXPHOS), and that venetoclax + aza targets LSCs by decreasing OXPHOS. Therefore, we tested the hypothesis that inferior responses of R/R patients to venetoclax + aza are due to changes in OXPHOS regulation in relapsed LSCs. LSCs were defined as cells bearing relatively low levels of reactive oxygen species (ROS-low), an effective means of enriching primary human LSCs.
We found that in contrast with untreated patients, venetoclax + aza does not decrease viability or OXPHOS in LSCs from R/R patients (Fig1). Furthermore, R/R LSCs had altered fatty acid metabolism that contributed to these OXPHOS differences, with increased flux of fatty acids into the TCA cycle (Fig 2). In addition, R/R samples compensated for the metabolic perturbations that occurred upon exposure to venetoclax + aza through upregulation of fatty acid uptake and metabolism into the TCA cycle (Fig 3).
Fatty acid metabolism is controlled by multiple genes and pathways. Integral to its activity is the gene Carnitine Palmitolytransferase 1 (CPT1), due to its pivotal role in the beta-oxidation of long chain fatty acids. Investigation of the Cancer Genome Atlas AML dataset reveals higher expression of CPT1 leads to significantly worse overall survival, suggesting increased fatty acid metabolism may drive a more resistant LSC population in R/R AML patients. We also found elevated baseline levels of CPT1 in patients who progressed on venetoclax + aza compared to those that had long term remissions (not shown). Therefore we utilized the CPT1 inhibitor etomoxir to block fatty acid metabolism. We found addition of etomoxir to cultures of R/R LSCs rescued the ability of venetoclax + aza to decrease OXPHOS and re-sensitized R/R LSCs to venetoclax + aza (Fig 4). To prove that this novel regimen targets functionally-defined R/R LSCs we performed ex vivo treatment followed by xenotransplantation of R/R patient specimens, which showed that upon etomoxir addition, engraftment potential is significantly decreased over venetoclax + aza alone (not shown).
Therefore we propose a novel mechanism for the increased resistance of R/R AML patients to venetoclax + aza involving altered energy metabolism. We find increased fatty acid metabolism in R/R patient specimens, and targeting this pathway using the CPT1 inhibitor etomoxir leads to sensitization to venetoclax + aza and rescued targeting of OXPHOS, allowing for LSC eradication.
Pollyea:Pfizer: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Argenx: Consultancy, Membership on an entity's Board of Directors or advisory committees; Celgene: Membership on an entity's Board of Directors or advisory committees; AbbVie: Consultancy, Research Funding; Agios: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Gilead: Consultancy; Celyad: Consultancy, Membership on an entity's Board of Directors or advisory committees; Curis: Membership on an entity's Board of Directors or advisory committees; Karyopharm: Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal